Hello, this is the J-BOX official store.
This article mainly introduces the materials released by the Ministry of Health, Labor and Welfare.
Hi, it seems that Takeda Pharmaceutical will release a new vaccine.
Licensed from Novavax, manufactured and distributed in Japan.
Before introducing a new vaccine, let's sort out the vaccine situation in Japan!

Please check the following table for the vaccine characteristics of each manufacturer.

Unlike Pfizer and Moderna's mRNA vaccine, Takeda's new vaccine (red frame) will be a recombinant protein vaccine.
What is a recombinant protein vaccine?
Based on the gene of the viral antigen ( SARS-CoV-2 spike protein), the vaccine is manufactured by converting the recombinant SARS-CoV-2 spike protein expressed using insect cells into nanoparticles. Adjuvants have been added to enhance the activation of Direct administration of viral proteins with an adjuvant can induce an immune response.
Recombinant protein vaccines are a type of inactivated vaccine, and are widely used technology, including hepatitis B virus vaccines. This technology is already widely used around the world and has a long history of use.



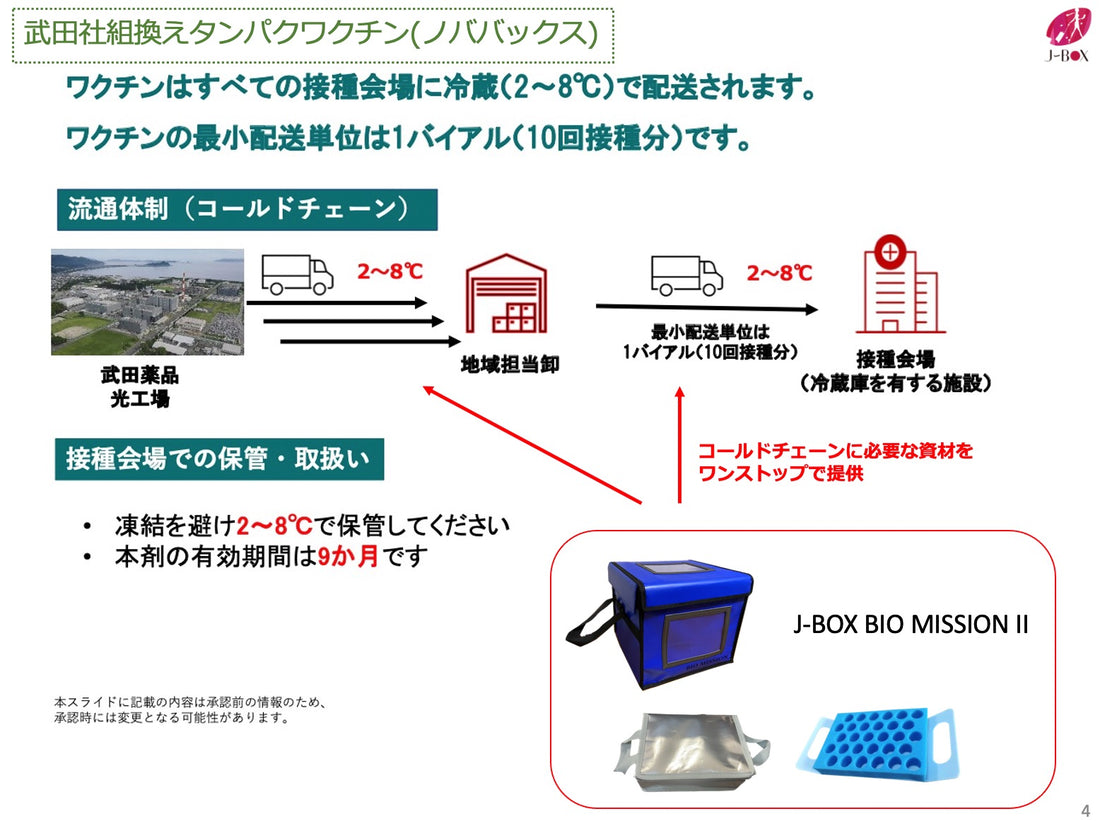
https://wish2020.jp/collections/bio-mission-m
★Both the vial holder and the inner bag are compatible with cooler bags procured by the Ministry of Health, Labor and Welfare.
Thank you for reading.
Stay tuned for next time! !